An Inkjet Print, As Seen Through a Scanning Electron Microscope
Today was awesome. I got the chance to use a fancy scanning electron microscope (SEM) at the University of Washington Nanotech User Facility thanks to the GEL 2011 Conference, Dee Breger, Alec Pakhomov, and Lindsey Maier .
What did I scan? Prints, of course! I took with me an inkjet print on Ilford Galerie Gold Fibre Silk, a carbon print, a salt print, and a cyanotype. We managed to scan the inkjet, carbon, and salt prints.
I was hoping to be able to see ink particles on paper, and I did! I was also hoping to get a good view of the carbon layers from the carbon print, but in hindsight we used the wrong part of the print as a sample so it was tough to see the carbon specifically, but we did see something else.
At this point you’re likely saying “this post is useless without photos!” and it is. So here’s photos. Lots and lots of photos.

Lindsey, sticking the inkjet sample onto one of the little sample posts prior to coating.

Three samples loaded into the chamber that will coat them with a gold/palladium so they scan properly. From left, clockwise, is the salt print, inkjet print, and carbon print.

The machine that does the coating. How can you not love a machine called a “sputter coater” that has a “sputter” setting? This machine was a little touchy. Lindsey explained that if you let the milliamps accidentally bounce off 50 you blow a fuse in the back of the machine and then have to scrounge to find a new one. She didn’t blow the fuse. Way to go Lindsey 🙂

The coating in progress. The purple glow is plasma in the vacuum chamber. She’s twiddling knobs and pressing buttons to kick the coating off.

The scanning electron microscope.

In hindsight I should have taken this photo with my finger in the picture, in the act of “pressing” the buttons 🙂

Loading the samples in the SEM. From left, clockwise, is salt (coated), salt (uncoated), carbon (uncoated), inkjet (coated), and carbon (coated). The uncoated ones were to see if there was enough silver or carbon inherent in the prints to scan without the coating, but they didn’t work well.

Always nice to see Microsoft represented 🙂 The software on the computer screen is what we used to do all the scanning.
Ok, so you’re probably saying “this thread is useless without pictures FROM THE FREAKING SEM!”. And you’re right. So here’s pictures from the SEM:

Ilford Galerie Gold Fibre Silk with a black and white image (printed in colour mode) from a Canon iPF5100. On the right is the paper edge where it was cut with scissors. On the left the things that look like ikura are ink blobs. What’s all that bright white stuff in the middle? More on that later…
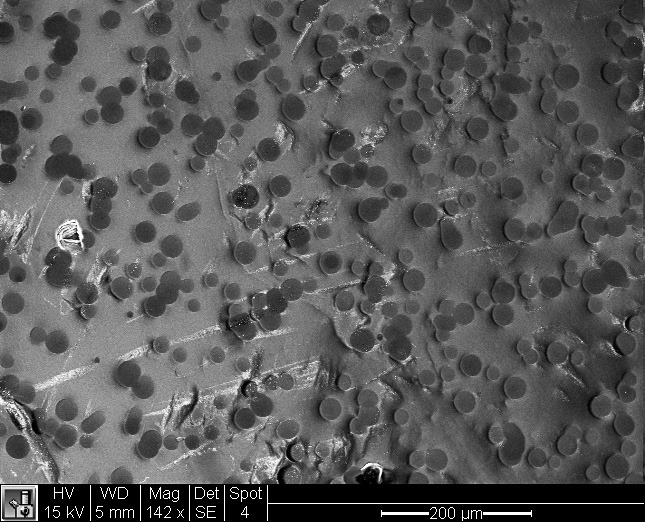
Here’s a better view of the ink on the paper. I’m assuming all the marks under it are light scratches on the surface of the paper.

Zooming in closer still to get a really good look at the ink dots. At this point we wondered what the ink was made of, so we switched over to another computer that can do a plot of the elements present. Here’s the plot:
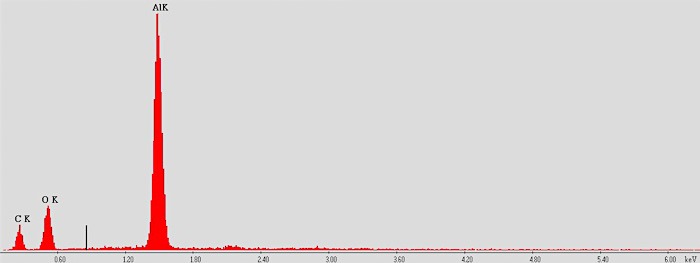
Looks like aluminum! We did a similar scan off one of the ink bubbles and got zero aluminum, so I’d say that’s pretty definitive.

This is a higher magnification view of the weird white stuff on the edge where we cut the paper. Unfortunately I don’t seem to have a screen shot of the element plot we did, but it came up with spikes of barium and oxygen. Baryta, anyone?

The salt print didn’t produce any images that were that interesting, but the above is from the carbon print. It’s the border between where the carbon image is and the base paper (top right). This is the first image we scanned and we couldn’t figure out what on earth these things were. I went back and looked at the original print and it was obvious: they’re bubbles. You can clearly see them all around the edge of the print. I imagine they were formed when the gelatin was being washed away.
The trip to the lab was awesome fun, and a huge thank you to Lindsey for patiently sitting with me to prep the paper and then drive the SEM as we nosed around the samples. Did I mention it was a ton of fun? Because it was!
Leave a Reply